|
Optics:
Light Sources, Filters, and Optical Instruments
Source:
Procedures in Experimental Physics
by John
Strong
Divisions of the
spectrum. The electromagnetic spectrum divides naturally into the
region for which the eye is sensitive, the infrared region, with frequencies
below those which we perceive as red, and the ultraviolet region, with
frequencies higher than those which we perceive as violet. These regions
are defined roughly by the wave lengths given in Table I. In the text
we will use microns for expressing wave length in the infrared and ngströms
for expressing wave length in the visible and ultraviolet. The visible
region includes less than one octave of frequency, while the so-called
infrared region embraces at least nine octaves and the ultraviolet embraces
five or six octaves.
 |
Light sources.
The sun. The sun naturally comes first in consideration of light
sources. Its use is recommended for many experiments because of its brightness
and because in the Fraunhofer lines it contains numerous convenient wave-length
landmarks. The Fraunhofer lines, which are conspicuous in the spectrum
exhibited by a good pocket spectroscope, are shown in Fig. 1.

Fig. 1. |
The energy distribution
in the solar spectrum, as observed through the atmosphere, is closely
approximated by that of a black body at 5400°K. The luminous efficiency
of the sun is about 80 lumens/watt. As will be seen in Fig. 2, this is
nearly as high an efficiency as it is possible to attain with a heated
body.

Fig.
2. |
A heliostat or coelostat
is required if a beam of sunlight is to be maintained in a fixed direction
in the laboratory. Heliostats are obtainable from scientific supply companies.
Their mirrors, which are usually silvered on the back, should be recoated
on the front surface with aluminum if it is desired to obtain in the reflected
sunlight the full range of solar spectrum down to the atmospheric cutoff
at approximately 3000 Angströms.

Fig. 3. |
The details of construction
for a home-made coelostat are: shown in Figs. 3 and 4. This coelostat
may be driven by the works of an alarm clock as shown; it may also be
driven by a Telechron clock. The secondary mirror of the coelostat has
controls operated by cords for making adjustments.
Tungsten lamps.
Tungsten lamps are the most convenient laboratory source of white light.
Their efficiency is about 11 lumens/watt for the nitrogen-coiled filament
type.

Fig.
4. |
The differences of
spectral energy distribution of various tungsten-filament lamps are illustrated
in Fig. 6, Chapter XI. The spectrum of emission of the filament is limited
in the ultraviolet and infrared by the transmission of glass. With glass
bulbs 1/4 mm in thickness, the spectrum extends from about 3100 Angströms
in the ultraviolet to 3 in
the infrared. Two tungsten lights convenient for many purposes in the
laboratory are shown in Fig. 5. The one shown on the left is a projection
lamp. It requires 6 volts and 18 amperes. An autotransformer or high-capacity
storage battery serves as power source. The battery is, of course, preferred
when constancy and steadiness of the emission are important.1
The lamp shown at the right has a straight filament. It is useful as a
galvanometer lamp. Both of these lamps are obtainable commercially.2 in
the infrared. Two tungsten lights convenient for many purposes in the
laboratory are shown in Fig. 5. The one shown on the left is a projection
lamp. It requires 6 volts and 18 amperes. An autotransformer or high-capacity
storage battery serves as power source. The battery is, of course, preferred
when constancy and steadiness of the emission are important.1
The lamp shown at the right has a straight filament. It is useful as a
galvanometer lamp. Both of these lamps are obtainable commercially.2

Fig. 5. |
A trade-mark on the
end of a tungsten lamp bulb, when it interferes with 'he light emission
of the filament, may be removed by polishing with rouge and felt or with
wet crocus cloth.
A lamp3
with a quartz bulb for absorption spectra is shown in Fig. 6. The bulb
contains argon at 1-1/2 atmospheres pressure. The tungsten operates at
about3100°C. and gives a continuous emission spectrum extending into
the ultraviolet to 2500 Angströms. At the operating temperature,
the vapor pressure of tungsten is appreciable, and it would normally blacken
the quartz part of the bulb. However, vertical convection currents of
argon gas carry the evaporated tungsten molecules upward from the filament,
so that they are not deposited on the quartz but rather on the upper glass
part of the bulb, where they do not impair the usefulness of the lamp.

Fig.
6. |
Welsbach mantle.4
This refractory mantle was formerly used extensively for house illumination
and is now used in gasoline lamps. It is brought to incandescence in the
outer hot surface zone of a Bunsen burner type of flame, where it assumes
a temperature nearly as high as the Bunsen flame temperature. The mantle
is composed of thorium oxide with 0.75 to 2.5 per cent cerium oxide added
to increase its visible emissivity. This addition of cerium oxide plays
much the same role as the sensitizer for a photographic plate; that is,
it introduces an absorption band in a desired spectral region without
materially affecting the optical properties elsewhere. The effect of the
cerium oxide is to make the emission in the green 30 percent greater than
that of a black body at 1800°C., whereas the emissions in the red
and blue correspond closely to 1800°C. color temperature.5
The near infrared emissivity is less than 1 per cent from 0.7 to about 6
to about 6 , and the incapacity
of the mantle to radiate heat in this important region accounts for its
high temperature. For the spectrum beyond 10 , and the incapacity
of the mantle to radiate heat in this important region accounts for its
high temperature. For the spectrum beyond 10 the mantle again has an emissivity greater than 75 per cent. The mantle
is an excellent laboratory source for those long wave-length infrared
radiations.6
the mantle again has an emissivity greater than 75 per cent. The mantle
is an excellent laboratory source for those long wave-length infrared
radiations.6
Barnes suggests heating
the mantle with a sharp oxygen flame striking it at a grazing angle.7
This gives it a higher temperature, and also the elongated heated section
produced is properly shaped for illuminating the slit of a spectrometer.
More recently, Pfund has devised an arrangement to combine both electric
and flame heating, allowing the attainment of even higher temperatures.8
Nernst glower.
Nernst filaments are composed of zirconium dioxide powder with about 15
per cent yttrium oxide powder.9 For operation on alternating
current, flexible platinum lead wires are later cemented to each end of
the refractory tube with a mixture of the oxide powders and zirconium
chloride as a binder. For operation on direct current, the manner of attaching
the electrodes is more complicated. The Nernst lamp normally operates
at around 2000°K. Its spectrum extends well into the ultraviolet and
infrared. However, beyond 15 its emission is said to be inferior to the emission of the Globar heater.
its emission is said to be inferior to the emission of the Globar heater.

Fig. 7. |
At one time the Nernst
glower offered great promise for commercial lighting, owing to a luminous
efficiency of 6 lumens/watt as compared with 3 lumens/watt for the carbon
filament. However, the modern incandescent lamp with a coiled tungsten
filament in an atmosphere of nitrogen, having an efficiency of 11 lumens/watt,
entirely changed matters. The use of the Nernst light is now confined
to the laboratory. Here its usefulness depends upon the fact that it is
operated in air and has a convenient form (cylinder 0.4 to 0.6 mm in diameter
and 1 to 2 cm long) for focusing on the slit of a spectrometer. Griffith
has described details of construction for making Nernst filaments.10
Since the Nernst
lamp has a negative temperature coefficient of resistance, it must be
stabilized with external resistance or, better, with a ballast lamp having
an iron-wire filament mounted in hydrogen.11 The iron wire
of this lamp runs at a faint red glow; its remarkable current-stabilizing
effect in an atmosphere of hydrogen at 30 to 100 millimeters pressure
is shown in Fig. 7. Such a ballast lamp consumes 10 or 15 per cent of
the total power needed for operating the Nernst filament.

Fig.
8. |
Globars. The
Globar is a rod of bonded silicon carbide about 5/16 inch in diameter
and about 10 inches long. The ends fit into aluminum cup electrodes. A
potential of 100 volts across the rod brings it to an orange or yellow
heat. It can be operated in air at a temperature above 1000°C., although
at temperatures around 2000°C. the carbide dissociates and carbon
is vaporized or oxidized, leaving silicon, or, in the presence of air,
silicon dioxide. A protective layer of thorium dioxide sintered to the
outside of the Globar with thorium chloride as binder will allow of temperatures
in excess of 2000°C.12 A suitable mounting for the Globar
is shown in Fig. 8.
Carbon arcs.
The carbon arc is useful as a laboratory light source. Ordinarily, the
positive carbon is mounted horizontally. An 8-mm positive carbon is consumed
at the same rate as a 6-mm vertical negative carbon. Accordingly, if carbons
of this size are used, they may be fed into the arc automatically by clockwork.
The carbon arc requires
at least 40 volts to operate it. Higher voltage increases the size of
the positive crater without materially affecting its surface temperature.

Fig. 9. |
The character of
the light emission from the ordinary carbon arc may be influenced by the
addition of metallic salts as cores in the carbons. (Magnesium fluoride
is often used to get a white arc.) The spectral distribution of the carbon
arc with cored carbons is illustrated in Fig. 9. This is a curve of galvanometer
deflections against wave length, as determined with a quartz monochromator
(shown in Fig. 32) and cesium oxide photocell. (See Chapter X.) The slit
widths were the same for all wave lengths. This curve does not correct
for the transmission of the image-forming lens (shown in Fig. 5, Chapter
XI) which was used to focus the light. Without this lens the spectrum
would have extended well into the ultraviolet.
The ordinary carbon
arc has a crater brightness of about 13,000 candles/cm2 and
an efficiency of about 35 lumens/watt. The Sperry Gyroscope Company has
produced an arc that uses special shields to confine the current to a
definite boundary around the rotating crater.13 This arc is
about six times as bright as the ordinary arc.

Fig.
10. |
Lummer has succeeded
in obtaining extreme temperatures in the carbon arc by operating it in
an inert atmosphere under high pressure. Under a pressure of 22 atmospheres
he was able to obtain temperatures of 7600°K., considerably in excess
of the solar surface temperature. The surface brightness reported for
this temperature was 280,000 candles/cm2. The attainment of
such temperatures and brightness is difficult.
A technique of
measuring. For the preliminary study of a spectrum plate, a technique
of measuring and recording data which is neat and avoids confusion is
illustrated in Fig. 10. This procedure employs an enlarged print of the
original spectrum plate to identify the iron or other reference lines
appearing in the eyepiece of the comparator. To facilitate this identification,
the wave lengths of the iron lines are written in the margin of the print.
Also, the print serves as a permanent record of the appearance of the
spectrum as well as a record of the data of measurement.
First, the wave lengths
of conspicuous iron comparison lines, which are to be used as reference
lines in the measurement, are written in the margin. The original plate
has the same appearance in the eyepiece of the comparator as the enlarged
print; thus it easily serves to identify the comparison lines. After the
wave length of each unknown line is determined by interpolation, it is
recorded on the clear margin of the print as shown at the left in Fig.
10. Notes may also be added in this margin when the wave lengths are later
identified by reference to Kayser's tables.14

Fig. 11. |
Iron arcs.
The iron arc is used in the laboratory by the spectroscopist as a source
of ultraviolet light and also as a standard comparison source. Its spectrum
has been thoroughly studied, and the wave lengths of the lines, as well
as the influence of pole and pressure effects on them, are well known.15
An iron arc developed
by Pfund16 suitable for use in the laboratory is shown in Fig.
11. An iron oxide bead is placed on the lower electrode for stabilizing
the arc. If the upper electrode is a graphite rod, the arc is even more
stable than it is with an iron electrode.17 The arc can be
started by rubbing a carbon across the gap.
Low-pressure mercury
arcs. The low-pressure mercury arc is a convenient laboratory light
source.18 It gives several strong lines in the visible, ultraviolet,
and near infrared spectra. These lines are far enough apart to be separated
with filters. (See Table XI.)
The ultraviolet spectrum
of the arc in a fused quartz tube extends to about 2000 Angströms.The
energy at the extreme short wave-length limit produces ozone in the air.
The ozone formation, however, becomes weaker and weaker as the lamp is
burned, owing to changes in the transmission limit of the quartz. Finally
the ozone formation practically ceases. Baly has found that such changed
quartz will emit a green phosphorescence and will regain its original
transparency if it is heated in the blast burner.19
The Cooper-Hewitt
type of mercury light has a brightness of about 2.3 candles/cm2.
The ordinary Cooper-Hewitt illuminating lamp has a tube 4 feet long and
about 1 inch in diameter. It is a convenient light source for many experiments
when an extended source is desired, as for observing Haidinger's and Newton's
fringes. To get uniform illumination over an extended area, drafting linen
is hung below the lamp.

Fig.
12. |
In glass the Cooper-Hewitt
lamp does not, of course, emit all of the ultraviolet spectrum. Recently
this arc has been put on the market, made with a tube of Corex red-purple
glass which suppresses the visible radiation (except 4046) and transmits
the near ultraviolet. In this form it is excellent for therapeutic use.
The commercial hot
quartz vacuum arc is much more brilliant (350 candles/cm2)
than the Cooper-Hewitt lamp discussed above. The ordinary hot quartz lamp
is not of a convenient form for use in the laboratory, but it is now available
in the form of a vertical straight quartz tube constructed especially
for laboratory use.20 These laboratory arcs are equipped with
rectifiers, so that they may be operated on either alternating or direct
current.
High-pressure
mercury arcs. Harries and Hippel21 have described a high-pressure
mercury lamp which is now commercially available.22 This is
illustrated by Fig. 12. The lamp is mounted in a nearly light-tight case,
a very convenient construction for use in the laboratory. The lamp is
made of uviol glass or quartz, with or without added cadmium to obtain
the red cadmium 6438 Angströms line. Schott glass filters are also
supplied for isolating the yellow, green, blue, violet, or ultraviolet
lines.
The spectrum of the
high-pressure lamp exhibits considerable continuous background. Accordingly,
the spectral purity obtainable with it by the use of filters is not as
great as it is with the low-pressure arc. The emission, however, is very
steady, especially when the lamp is operated on storage batteries.

Fig. 13. |
Cornelius Bol of
Stanford University (formerly of the Philips Laboratory, Eindhoven, Holland)
has developed a so-called super-high-pressure mercury arc.23
The discharge which produces the high pressure is started, however, by
argon at a pressure of 2 or 3 cm of mercury. The operating potential for
the lamp is around 500 volts. Heat generated e by the argon discharge
volatilizes the liquid mercury exposed in the lamp until a pressure of
mercury gas of about 200 atmospheres is attained. On account of the high
ultimate pressure, the lamp must be made of a thick-walled capillary tube
as shown in Fig. 13. The tungsten electrodes project beyond the reserve
mercury in order to guide the discharge down the central part of the tube.
In the center, temperatures of 8600°C. and brightness values several
times greater than the brightness of molten tungsten are attained. For
example, a lamp operating on 640 volts at a pressure of 200 atmospheres
has a brightness of 180,000 candles/cm2 and a luminous efficiency
of 79 lumens/watt. The emission of a Bol lamp is shown in Fig. 9. (See
also Table II.)
 |
The inside surface
of the quartz capillary probably attains a temperature in excess of the
critical temperature of mercury, so that no liquid mercury can condense.
The mercury gas envelope around the hot central core of the arc absorbs
the resonance line emitted in the core, and at the obtaining pressure
and temperature the resonance line is so broad that its absorption extends
over the major part of the ultraviolet spectrum (to 2700 Angströms).
The electrodes are
sealed in the Bol lamp with a new glass. A lamp of convenient size for
use in the laboratory has the electrodes spaced 1 cm apart. It is first
filled with 2 cm pressure of gaseous argon and then with liquid mercury
until the 30-mil tungsten wires project about 1/2 mm beyond the mercury
at each end. A 640-volt transformer is suitable for operating the light.
It is connected in series with the arc and a suitable choke coil. When
the arc is shorted out, the choke will draw about 3.4 amperes from the
transformer.24

Fig.
14. |
A "cold," low-pressure
mercury-vapor lamp is shown in Fig. 14.25 This lamp employs
a few millimeters pressure of hydrogen, argon, or one of the other noble
gases as a starting gas. Heat developed by the discharge in the noble
gas soon distills mercury vapor from small globules of the liquid metal.
The potential for operating the lamp is obtained from a sign transformer
or from a storage battery and spark coil. This lamp is only about one
tenth as brilliant in the visible as the Harries and Hippel lamp, but
its emission at 2536 Angströms is
many-fold greater. In fact, about 80 per cent of its total emission is
in the resonance line.
The resonance line
from the mercury lamp shown in Fig. 14 is so strong that the mercury vapors,
rising from a globule of liquid mercury held in the hand, cast a strong
shadow on a fluorescent screen.26 With a 3-mm Corex red-purple
filter to suppress the visible spectrum, this lamp is ideal for exciting
the fluorescence of minerals.
This type of mercury
light is very useful in the laboratory. When neon is used instead of argon
as the starting gas, this single source yields a series of strong lines
well distributed over the spectral range from 2536 to 10,140 Angströms.
The gap in the mercury spectrum between 6907 and 10,140 Angströms
is filled by a series of neon lines around 8300 Angströms.27
Filters for use with
the various mercury arcs to yield monochromatic light are discussed in
a later section.
Other gaseous
discharges. Commercial sodium arcs are now available. They are confined
in a special glass container that is not attacked by the metal vapor.28
These arcs operate inside a clear Dewar flask and afford a large-area
source of monochromatic light which is particularly suited to many laboratory
tests and demonstrations. The characteristics of this and the Bol lamp
are given in Table II.
Pyrex is not attacked
by sodium as readily as are soft glasses, and by fusing borax or boric
acid to the inside surface, its resistance to the alkali metal can be
further increased.29 Magnesia crystals are not attacked by
vapors of the alkali metal, and they may be used for experiments in which
sodium, at higher temperatures and pressures, is to be confined behind
windows transparent to both the ultraviolet spectrum and the infrared
spectrum.30

Fig.
15. |
The ultraviolet spectrum
obtained from a hydrogen discharge tube- is continuous, extending from
the short wavelength emission limit of incandescent tungsten toward shorter
wave lengths to the limit of transmission of quartz. This hydrogen continuum
is most effectively excited by sources of the type developed by Duffendack
and Manley, Smith and Fowler, Munch, and Jacobi.31 These sources
excite the spectrum with thermoelectrons emitted from a hot cathode.
Capillary discharge
tubes filled with many different elementary gases are now available commercially.32

Fig.
16. |
Sparks. To
obtain the spark spectrum characteristic of the materials composing the
electrodes, it is necessary to use a condenser of sufficient capacity
to give an explosively noisy spark. Either a transformer or an induction
coil can be used as the source of potential. A spark between magnesium
electrodes, especially if it is confined between glass plates, is very
brilliant. Such a light source, shown in Fig. 15, is useful for shadow
photographs of bullets in motion, and so forth, and for the photography
of sound waves by the Schlieren-methode.33 The duration of
the illumination from the magnesium spark can be made extremely short.
Flames. Flames
such as the Bunsen flame, which are almost colorless, give characteristic
emission spectra when volatile metallic vapors are introduced. The metals
most commonly used to obtain monochromatic or nearly monochromatic light
are given in Table III.
 |
Sodium light may
be obtained by wrapping asbestos, soaked in sodium chloride, around the
tip of the Bunsen burner tube. Another method of introducing the salts
into the flame is illustrated by the device shown in Fig. 16. A neodymium
filter may be used to absorb the emission of sodium vapor and at the same
time transmit the red emission lines from potassium or lithium vapors.
To obtain the metallic thallium spectrum, a bead of the metal, fused in
a platinum-wire loop, is touched to the edge of the Bunsen flame. The
bead is introduced just far enough to obtain the desired rate of evaporation.
If the bead is held too far inside the flame, it boils away rapidly. Inasmuch
as thallium is a poisonous metal, a high concentration of the vapors in
the room should not be allowed. Also, sodium, potassium, and lithium vapors
may be introduced into a Meker burner flame by placing a small globule
of fused sodium chloride, potassium chloride, or lithium chloride on the
grill of the Meker burner.
 |
The
ultraviolet. The portion of the ultraviolet spectrum treated here
will be limited to the wave-length range 2000 to 4000 Angströms.34
In the long-wave half of this region between 4000 and 3000 Angströms
many substances are transparent, including mica, celluloid, diamond, Canada
balsam, ether, glycerin, acetone, turpentine, xylene, and in thin layers,
many ordinary glasses. (See Table IV.) For the entire region from 4000
to 2000 Angströms the list of materials is not so great. It includes
rock salt, potassium chloride, fluorite, magnesia, lithium fluoride, alum,
gypsum, sugar, calc-spar, water, ethyl alcohol, glacial acetic acid, liquid
ammonia, fused and crystalline quartz, and cellophane. (For the transmission
of cellophane see Table V.)
 |
Prisms, lenses,
and mirrors for the ultraviolet. Only a few of the substances mentioned
above are suitable for making prisms and lenses. Fluorite and quartz make
excellent prisms. They can be combined to make achromatic lenses. But
the scarcity of fluorite of good optical quality in large sizes makes
these achromats very expensive. A combination of quartz and rock salt
is sometimes used for making achromats. Recently, synthetic alkali halides
and magnesium oxide have become available in large pieces, and these,
together with other synthetic substances, will no doubt become important
for constructing ultraviolet optics. The optical constants of some of
these materials for the visible spectrum are given in Table VI.
Concave aluminized
mirrors are now used for ultraviolet optical systems. They have the same
focus for ultraviolet as for visible light, and therefore they can be
adjusted visually.
Filters for the
ultraviolet. Thin metal films are among the most interesting filters
for the ultraviolet. The transmission band exhibited by silver and the
alkali metals is associated with a gap lying between the region where
the reflection is ascribed to the effect of free electrons (on the long
wave-length side of the gap), and the region where reflection is ascribed
to bound electrons (on the short wavelength side). In silver, this gap
at 3160 Angströms is approximately 100 Angströms wide. It is
much wider than this for the alkali metal films.

Fig. 17. Transmission
of a potassium film.36 |
Potassium films may
be used as a filter for isolating ultraviolet radiations. The full transmission
of potassium in the ultraviolet begins at 3000 Angströms for films
of a thickness just sufficient to be opaque in the visible to sunlight.
R. W. Wood has studied this phenomenon and describes how these films can
be formed on a quartz-glass bulb cooled to liquid air temperatures.35
Unfortunately, films prepared as he describes are only permanent at temperatures
considerably below room temperature. O'Bryan, however, has shown how potassium
may be deposited between quartz-glass plates to give films permanent even
at the elevated temperature of boiling water.36 The transmission
of these thicker films begins at about 3350 Angströms, becomes about
25 per cent at 2500 Angströms, and decreases to a little below this
value as the wave length 2000 Angströms is approached. The transmission
of such a potassium film is illustrated by Fig. 17.
Bromine vapor can
also be used as a filter. It is transparent to the ultraviolet rays. A
layer of saturated bromine vapor 5 cm thick at room temperature is opaque
to blue light and nearly opaque to green light, as one can readily see
by interposing a bottle containing a little liquid bromine between a mercury
lamp and a pocket spectroscope. The ultraviolet transmission of bromine
begins at 3800 Angströms, and the vapor is quite transparent to the
spectrum from wave length 3500 Angströms down to at least 2345 Angströms.

Fig.
18. Transmission spectra of various materials. After
Williamson, R.C., Phys. Rev., 21, 111 (1923). |
A 5-mm layer of a
solution of nitrosodimethylanalin (10 mg to 100 cc water) has about the
same transparency as the bromine vapor.37
A filter of 14 g
pure, iron-free nickel sulphate crystals and 10 g pure cobalt sulphate
crystals dissolved in 100 cc distilled water is opaque to the visible
spectrum but transparent in the ultraviolet below 3300 Angströms.
In layers 3 cm thick this filter transmits 3.5 per cent of the 3342 Angströms
mercury line and 96 per cent of 3126 Angströms line, and it is transparent
as far down in the ultraviolet as 2300 Angströms.38
The ultraviolet transmission
limit for mica is at about 2800 Angströms for
0.01 mm thickness. Mica of this thickness is completely opaque at wave
lengths below 2600 Angströms.

Fig. 19. Transmission
of various liquids.
After Brode, W.R., J. Phys. Chem., 30, 56, (1926) |
The transmissions
in the ultraviolet of some other materials are illustrated in Figs. 18
and 19.
Polarization of
the ultraviolet. The new sheet polarizers39 made of herapathite
are opaque to ultraviolet light. (See Fig. 38.) Although the calcite of
Nicol prisms is transparent to 2000 Angströms, the Canada balsam
used for cementing them is not transparent in the ultraviolet at wave
lengths below about 3000 Angströms. For cementing optical surfaces
to be used in the ultraviolet, glycerin, castor oil, or dextrose sugar
should be used. A Wollaston prism may be used to polarize light in the
ultraviolet when its parts are properly cemented.
The infrared.
The infrared spectrum extends from 7600 Angströms, or 0.76 ,
to about 400 ,
to about 400 . A thermopile
or radiometer is generally used for measuring infrared radiation. As the
operation of these instruments depends on thermal effects produced by
the radiation, the infrared spectrum is often referred to as the heat
spectrum. The infrared radiations are emitted by heated bodies. Ordinarily,
heated bodies are used as laboratory sources for the infrared spectrum. . A thermopile
or radiometer is generally used for measuring infrared radiation. As the
operation of these instruments depends on thermal effects produced by
the radiation, the infrared spectrum is often referred to as the heat
spectrum. The infrared radiations are emitted by heated bodies. Ordinarily,
heated bodies are used as laboratory sources for the infrared spectrum.
It is convenient
to divide the heat spectrum into three regions: The near infrared, from
1.1 to 20 to 20 ;
the intermediate infrared, from 20 ;
the intermediate infrared, from 20 to 40
to 40 ; and the far infrared,
from 40 ; and the far infrared,
from 40 to 400 to 400 .
The spectroscopic significance of the near infrared is that the characteristic
frequencies of gases which fall in this region generally arise from molecular
oscillations, whereas the characteristic frequencies which fall in the
visible and ultraviolet regions arise in general from electronic oscillations.
On the other hand, in the far infrared the characteristic frequencies
of gases arise from molecular rotation and molecular bending. In the case
of crystals the characteristic frequencies in the near infrared are generally
interatomic oscillations within the chemical radicals that exist as units
in the crystal, while frequencies in the far infrared are due to oscillations
of the positive ions (or radicals) of the crystals relative to the negative
ones. .
The spectroscopic significance of the near infrared is that the characteristic
frequencies of gases which fall in this region generally arise from molecular
oscillations, whereas the characteristic frequencies which fall in the
visible and ultraviolet regions arise in general from electronic oscillations.
On the other hand, in the far infrared the characteristic frequencies
of gases arise from molecular rotation and molecular bending. In the case
of crystals the characteristic frequencies in the near infrared are generally
interatomic oscillations within the chemical radicals that exist as units
in the crystal, while frequencies in the far infrared are due to oscillations
of the positive ions (or radicals) of the crystals relative to the negative
ones.
The intermediate
infrared spectral region from 20 to 40
to 40 was formerly closed to
investigation on account of the lack of transparent substances to be used
for making windows and prisms. There are now available, however, a transparent
paraffin of high melting point,40 and large synthetic crystals
of the alkali halides which are transparent in the range 20 was formerly closed to
investigation on account of the lack of transparent substances to be used
for making windows and prisms. There are now available, however, a transparent
paraffin of high melting point,40 and large synthetic crystals
of the alkali halides which are transparent in the range 20 to 40
to 40 .41 .41

|
Prisms, windows,
lenses, and mirrors for the infrared. The important prism materials
for the infrared are listed in Table VII. These materials are not ordinarily
combined to form achromatic lenses for focusing the infrared rays; mirrors
which are much more satisfactory are used. Even spherical mirrors are
useful for the less exacting work, since the slits in infrared spectroscopy
can never be set as fine as they can in the other spectral regions, in
which photography can be applied.42

Fig.
20. After
Barnes, R.B., and Bonner, L.G., J.O.S.A., 26, 433 (1936). |
Materials useful
for windows on absorption cells and vacuum radiometric devices are listed
in Table I, Chapter VIII. (See also Figs. 20, 21, 22.) Of these materials
the high-melting-point paraffin is of special interest, since it is one
of the few materials opaque to the near infrared spectrum and transparent
to the long wave lengths. Soot is another such material. Although it is
quite opaque in the visible, soot is translucent for the heat spectrum.

Fig. 21.
After Barnes, R.B., and Bonner, L.G., J.O.S.A., 26, 433 (1936). |
The reflection of
most metals such as silver, speculum, and aluminum is high in the infrared.
The reflectivity for wave lengths longer than about 10 can be calculated from the electrical conductivity of the metal by the
expression
can be calculated from the electrical conductivity of the metal by the
expression
 (1)
(1)
where  is in ohms mm2/m and
is in ohms mm2/m and  is in microns.
is in microns.

Fig.
22. Infrared transmission and reflection of quartz. After
A.H. Pfund. |
Reflection of
crystals. Residual rays. Crystals exhibit so-called bands of "metallic"
reflection at certain wave lengths where the reflection coefficient, usually
of the order of 5 per cent, approaches 100 per cent. This property of
crystals was first observed by E. F. Nichols.43 The bands of
high reflectivity exhibited by quartz, for example, are shown in Fig.
23 Quartz (for the ordinary ray) exhibits two strong bands, one at 8.9 and one at 20.8
and one at 20.8 . Rock salt
has only one band, at 52 . Rock salt
has only one band, at 52 . .

Fig. 23. After
H. Ruebens. |
Multiple reflections
from crystals are employed to isolate narrow bands of monochromatic radiation
from the heat spectrum. For example, if the spectrum from a heated body
is reflected once from a rock-salt crystal surface, the energy at wave
lengths about 52 are reflected
while those radiations elsewhere, especially in the short-wave spectrum,
where the reflection is nonmetallic, are attenuated about twenty times.
In spite of this attenuation by a single reflection, the energy in the
52 are reflected
while those radiations elsewhere, especially in the short-wave spectrum,
where the reflection is nonmetallic, are attenuated about twenty times.
In spite of this attenuation by a single reflection, the energy in the
52 band may still be much less
than the integrated energy reflected at other wave lengths. After a second
reflection, however, the short-wave spectrum is again attenuated about
twenty times, or four hundred times altogether, while the energy in the
band of waves around 52 band may still be much less
than the integrated energy reflected at other wave lengths. After a second
reflection, however, the short-wave spectrum is again attenuated about
twenty times, or four hundred times altogether, while the energy in the
band of waves around 52 is
little affected. Accordingly, after four or five reflections the only
radiations remaining, the so-called residual rays, are those of the 52 is
little affected. Accordingly, after four or five reflections the only
radiations remaining, the so-called residual rays, are those of the 52 band.
band.
 |
The use of these
successive reflections is a standard procedure for obtaining monochromatic
bands of radiation in the far infrared. The crystals used for obtaining
various wave lengths are listed in Table VIII. We shall describe the apparatus
used for obtaining residual rays in a later part of this chapter.

Fig.
24. |
Special absorbers
for the near infrared. Water is transparent from wave lengths greater
than 0.2 in the ultraviolet
throughout the visible spectrum. (See Fig. 24.) However, it is opaque
in the heat spectrum for all rays beyond the limits in the ultraviolet
throughout the visible spectrum. (See Fig. 24.) However, it is opaque
in the heat spectrum for all rays beyond the limits  for thickness
for thickness 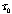 , as given in
Table IX. , as given in
Table IX.
A water filter is
often used to absorb the heat rays that are emitted when a carbon arc,
the sun, or a tungsten lamp is used as a light source. The use of a water
filter prevents the cracking of lantern slides with heat, burning of photographic
film, overheating of microscope objectives, or excessive heating of polarizing
Nicols.
The addition of cupric
salts to water results in increased absorption of the infrared. The absorption
for the infrared is illustrated in Fig. 24 for a 2-cm cell containing
cupric chloride.44
Manufactured glass
filters such as Aklo glass and the Schott filters BG17 and BG19 are designed
to remove the heat spectrum.45 (See Table X and also Jena Colored
Optical Filter Glasses, obtainable from Fish-Schurman Corporation, 250
East 43rd Street, New York City.) The transmission of BG17, 1 mm thick,
and BG19, 4 mm thick, is about the same as that of 2-cm of a nearly saturated
copper sulphate solution.
Visible spectrum.
Glass and gelatin filters are used for isolation of the mercury lines
They are easier to handle and much more permanent than water solutions.
The transmissions of some of the glass and gelatin filters commercially
available in this country are illustrated in Figs. 25 and 26. A list of
the filter combinations for the separation of various spectrum lines is
given in Table XI.

Fig. 25. Transmission
of glass and Wratten filters. |
The Christiansen
filter. The Christiansen filter consists of a mass of solid particles
immersed in a liquid medium, as, for example, particles of borosilicate
glass immersed in carbon disulphide and bezene.46 Fig. 27 shows
the dispersion for a borosilicate crown glass and for a 10 per cent solution
(by volume) of carbon disulphidein benzene (both anhydrous) at 20°C.
The filter composed of these two transmits freely the color for which
the indices of refraction of the liquid and solid phases are identical,
that is, where the two lines in Fig. 27 cross. For this color the medium
is optically homogenous. The filter is a nonhomogenous optical medium
for all wave lenghths. Accordingly, they are scattered. By means of the
arrangement shown in Fig. 28, the scattered waves are isolated from the
freely transmitted color. The individual transmissions of five filters
are shown in Fig. 29. These filters are 18 mm thick and were made up from
borosilicate glass using different concentrations of carbon disulphide
and bezene.

Fig.
26. From Glass Color Filters, Corning Glass Works, Corning,
New York. |
One limitation of
the Christiansen filter lies in its lack of complete opacity to wave lengths
on either side of the transmitted band.
This limitation is
a serious one. For example, when the filter is to be used in conjunction
with a highly selective receiver, such as a photocell, the response of
the receiver for rays weakly transmitted by the filter but for which the
receiver is exceptionally sensitive (or for which the emission of the
source is especially strong) may seriously interfere with the interpretation
of the results obtained. Another limitation of this filter is its sensitivity
to temperature changes. The filter cannot be used effectively in an intense
beam of light such as sunlight, owing to temperature gradients set up
in the cell.

Fig. 27.
After McAlister.
(See footnote 46.) |
However, the dependence
of the transmitted wave length upon temperature may be put to use. F.
Weigert and collaborators have found, for example, that a cell made of
particles of crown glass immersed in liquid methyl benzoate transmitted
red light at 18°C. (64°F.) and blue light at 50°C. (122°F.).47

Fig. 28. After
McAlister.46 |
A very interesting
Christiansen filter effect is exhibited by the infrared transmission of
thin powder films.48 Their maximum of transmission occurs at
the wave length at which the index of the powder is unity or equal to
the index of the surrounding medium. For magnesia this transmission maximum
in air is at 12.2 , and if the
filter is immersed in carbon tetrachloride, the maximum shifts to wave
length 9 , and if the
filter is immersed in carbon tetrachloride, the maximum shifts to wave
length 9 , at which both the
carbon tetrachloride and the magnesia have the same index. , at which both the
carbon tetrachloride and the magnesia have the same index.

Fig.
29. After McAlister.46 |
Reflection of
metals. Of the metals useful in the visible spectrum for reflection
of light, the three most important are aluminum, speculum, and silver.
Their reflectivities are shown in Fig. 30. It is to be noted that aluminum
is superior to newly deposited silver for all wave lengths less than 4100
Angströms. In the visible spectrum the use of aluminum instead of
silver is recommended. Although new silver has a better reflectivity in
the visible spectrum than aluminum, it soon tarnishes.

Fig. 30. |
The apparatus shown
in Fig. 31 was used for the above reflectivity measurements. This apparatus
measures the square of the absolute reflectivity directly (putting the
comparison mirror in both the numerator and denominator, so to speak).
Monochromators.
The best method of isolating a narrow wave-length band of high spectral
purity from a source of white light is to use a double monochromator,
that is, two single monochromators built together. High spectral purity
is often desirable for highly selective effects, such as, for example,
the determination of the long wave-length limit of the photoelectric effect,
or in any other case when the slight spectral impurity that one might
have with a single monochromator would vitiate the results of the measurement.
A step can be made in the direction of high spectral purity by the use
of filters in series with a single monochromator. These filters are, however,
usually less efficient than a single monochromator. The transmission of
a single monochromator is about 45 per cent.

Fig.
31. |
The monochromator
may have achromatic lenses, but these are very expensive if they are constructed
of materials which will function in the ultraviolet. Generally, monochromators
employ quartz lenses. These are brought to focus with a mechanism operated
by the wave-length drum. Fig. 32 shows how this is accomplished in the
Hilger-Müller double monochromator by the use of a cam bar mounted
on the prism table. As the prism table and lens system move as a unit
toward the slit system, the lenses are brought into focus for shorter
and shorter wave lengths. The cam bar is so constructed that it causes
the wave lengths to fall on the exit slits for which the lenses are in
focus.

Fig. 32. Hilger-Müller
double monochromator |
Use of mirrors
in monochromators. Parabolic mirrors are often used in monochromators,
because an optical system using mirrors is achromatic. However, mirrors
have the distinct disadvantage as compared with lenses that the parallel
collimated beam is returned in the direction of the entrance slit, a direction
which precludes a neat simple arrangement of the other optical parts.
To use a mirror on its optical axis requires either an auxiliary flat
as in the Pfund49 arrangement shown in Fig. 33 (a) or an off-axis
mirror as shown in Fig. 33(b). One way to make such an off-axis mirror
is to construct a large ordinary paraboloidal mirror and cut out the desired
mirror from one side of it.

Fig.
33. |
A mirror system composed
of spherical mirrors like the one shown in Fig. 33(c) may be used. This,
of course, introduces large distortions in the wave front. It is possible,
however, by proper orientation of a similarly imperfect mirror to compensate
in a measure for the distortions produced by the collimator and to obtain
better definition than would be possible even if a perfect telescope were
used. The proper arrangement of the telescope system for achieving this
compensation is shown in Fig. 34, with the regular Wadsworth arrangement.50
The optical train
in monochromators is usually either the Littrow arrangement or the Wadsworth
arrangement, both of which use the prism at minimum deviation. These arrangements
are shown in Fig. 35.51

Fig. 34. |
Water monochromator.
An ultraviolet monochromator with improvised optics, devised by Harrison,52
is shown in Fig. 36. The optical parts consist of a water prism and a
spherical aluminized mirror. This monochromator is very simple, and optically
it is good enough for isolating the stronger mercury lines (as the illustration
of the produced spectrum shows). It has a relatively high aperture, f/6.
The dispersions of crystal quartz, fused quartz, and water are related
as 25:21:19 at 3000 Angströms. Since water is more transparent to
the ultraviolet than quartz, this monochromator can be used for isolating
wave lengths as short as 1820 Angströms.

Fig.
35. |
Focal isolation.
Fig. 37 shows the method of focal isolation invented by Wood to isolate
the far infrared radiations from a Welsbach burner.53 When
the first lens is positioned in relation to the light source at a distance
equal to twice its focal length for the far infrared rays, where the index
of refraction is 2.25, the near infrared rays emerging from the lens are
divergent. An opaque spot at the center of the quartz lens prevents the
direct transmission of the median near infrared rays through the aperture
provided at the focus of the far infrared rays. Usually two lenses are
arranged in series of effect complete separation of the far infrared rays.

Fig. 36. |
A focal isolation
method has been applied to the isolation of the 1940 A group of aluminum
lines with a quartz lens.54 And, while there is for quartz
no such diversity of index in this part of the spectrum as there is in
the infrared, yet these lines are separated from the rest of the aluminum
spectrum with a spectral purity of 0.98. The intensity obtained is sevenfold
greater than that obtainable from a quartz monochromator. This focal isolation
method has also been applied to the 2030 Angströms to 2140 Angströms
group of zinc lines.
Residual-ray isolation.
Apparatus using the residual-ray method for the isolation of wave lengths
in the infrared is illustrated in Fig 38.55 The apparatus shown
at the top of this figure employs four crystal reflections, while the
one at the bottom, placed at the focus of an image-forming mirror, uses
only two crystal reflections.

Fig.
37. |
When the two-crystal
apparatus is equipped for 6.7 (crystals of calcite) it is useful for measuring humidity, since this
region of the spectrum is very sensitive to moisture in the radiation
path. On the other hand, with either quartz, Carborundum, or potassium
chromate crystals, which give bands of radiation at 8.7
(crystals of calcite) it is useful for measuring humidity, since this
region of the spectrum is very sensitive to moisture in the radiation
path. On the other hand, with either quartz, Carborundum, or potassium
chromate crystals, which give bands of radiation at 8.7 ,
12 ,
12 , and 11.6 , and 11.6 ,
respectively, the instrument is useful as a radiation pyrometer insensitive
both to water vapor and to light smoke or haze. In the region from 8 ,
respectively, the instrument is useful as a radiation pyrometer insensitive
both to water vapor and to light smoke or haze. In the region from 8 to 13
to 13 there is very little
absorption by the water in the air even when it is humid; in this region
of the spectrum the entire thickness of the atmosphere exhibits a transmission
comparable to the transmission of the atmosphere for green and yellow
light (T = 85 per cent). there is very little
absorption by the water in the air even when it is humid; in this region
of the spectrum the entire thickness of the atmosphere exhibits a transmission
comparable to the transmission of the atmosphere for green and yellow
light (T = 85 per cent).

Fig. 38. |
Polarization.
There are now new polarizers available for use in the visible spectrum,
but they are not as efficient as Nicol prisms.56 The transmission
of these polarizers, shown in Fig. 39, does not yield as high efficiency
as that of a Nicol prism. For plane-polarized light of proper azimuth
a Nicol prism transmits about 80 per cent. Two Nicol prisms in series
transmit a maximum of about 32 per cent of unpolarized white light. At
the other extreme, two Nicol prisms accurately crossed are quite opaque.
For example, they will not transmit enough sunlight to make the disk of
the sun discernible. However, to obtain this degree of, opacity, the Nicol
prisms must be crossed very precisely (to an accuracy of the order of
1 second of arc).
The new polarizers
have the advantage over Nicol prisms that they can polarize a beam of
greater aperture (both areal and angular). Two applications of the new
polarizers are illustrated in Figs. 40 and 41.

Fig. 39. |
One of these, illustrated
in Fig. 40, applies to the measurement of strain in glass. Objects to
be tested for strain, as, for example, glass-to-metal seals, are immersed
in a jar fitted with parallel glass sides and containing a liquid medium
having the same index of refraction as the glass. This medium may, for
example, be a mixture of the proper proportions of carbon disulphide and
benzene or a mixture of zylene and alcohol. Polarized light obtained from
a lamp by reflection off black glass at the polarizing angle (or reflection
off the back of an exposed photographic plate which has been developed,
fixed, and dried) is viewed through a full-wave mica plate and analyzer.
(The construction of the full-wave plate is described below.) When a full-wave
plate is placed in front of the analyzer, slight variations of the polarization
over the field of view are manifest as variations of color from the purple
of the unstressed condition.

Fig. 40. |
Engineering applications
of polarized light. The property of isotropic transparent materials
that a strain makes them double refracting is used by engineers for studying
the magnitude and distribution of stress produced by loading various two-dimensional
structures, such as, for example, the shapes represented by the cross
section of a dam.57 An arrangement for such studies using spherical
mirrors and the new polarizers is shown in Fig. 41. The astigmatism (due
to using the mirrors off axis) can be balanced out, at least in part,
by tipping the camera lens about a horizontal axis by a suitable amount.
The model of the shape to be tested is usually made from a clear sheet
of Bakelite or Marblette. Table XII gives the coefficient of forced double
refraction for various materials suitable for constructing models.

Fig.
41. |
The quarter-wave
plates are used in the illustrated arrangement to allow the elimination
of the pattern of isoclinics (the lines along which the principal stress
in the specimen has a constant inclination) from the pattern of isochromatics
(the lines along which the quantity (p-q) has a constant value).
Here p and q are the principal stresses produced in the model by the applied
loading Methods of determining the magnitude of the quantities p
and q from the measured isoclinics and isochromatics cannot be
described here, since they are quite complicated.58 However,
in spite of this, the experimental method of studying the stresses in
many structures is easier than the theoretical method, and the experimental
method has the advantage over the theoretical method that it carries with
it the conviction of a more direct appeal to nature for the information
desired.
Quarter-, half-,
and full-wave plates. Quarter-, half-, and full-wave plates are made
of quartz, selenite, or mica cut or split parallel to the optical axis.
The thickness of the plate is made such that the relative retardation
of the ordinary and extraordinary ray is 1/4, 1/2, or 1 full wave length.
The thickness  required for
a quarter-wave plate is required for
a quarter-wave plate is
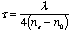 (2)
(2)
where  is the index of the crystal for the extraordinary ray and
is the index of the crystal for the extraordinary ray and 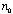 for the ordinary, and
for the ordinary, and  is the
wave length in question. For mica the thickness of a quarter-wave plate
for the D lines is about 0.036 mm. Although for mica the quantity
( is the
wave length in question. For mica the thickness of a quarter-wave plate
for the D lines is about 0.036 mm. Although for mica the quantity
( - -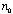 )
varies from specimen to specimen,59 it can be taken as essentially
constant for all wave lengths. Therefore, the thickness of a quarter-wave
plate is roughly proportional to the wave length for which it is intended. )
varies from specimen to specimen,59 it can be taken as essentially
constant for all wave lengths. Therefore, the thickness of a quarter-wave
plate is roughly proportional to the wave length for which it is intended.
 |
A quarter-wave plate,
when it is set perpendicular to a beam of polarized light with its principal
directions at 45° to the azimuth of polarization, retards one half
of the polarized light until its phase is 90° behind the phase of
the other half, thus producing circular polarized light. Conversely, a
quarter-wave plate will change circular to plane-polarized light. A half-wave
plate, similarly oriented, transforms plane-polarized light to plane-polarized
light rotated in azimuth by 90°.
The principal directions
of mica are determined by interposing it between crossed Nicols. The principal
directions are parallel and at right angles to the azimuth of polarization
of the incident light when the mica (of any thickness) is so oriented
that it does not affect the cutoff of the second Nicol.
Tutton's test60
for distinguishing between the two principal directions in a quarter-wave
plate is to place the plate between crossed Nicols (with its plane perpendicular
to the axis of the beam of incident white polarized light) oriented in
an azimuth such that the restored light is a maximum. The principal directions
in the plate now make angles of 45° with the azimuth of vibration
of the incident polarized light. The mica plate is rotated first about
one principal direction and then about the other, so that, in each case,
light traverses a thicker layer of mica. In one case the color passes
from bluish gray through iron gray to black, and in the other case the
color passes from white to yellow and then through colors of a higher
order. The latter color sequence corresponds to rotation about the principal
direction of slower vibration in the mica and the first case corresponds
to the principal direction of faster vibration in the mica.
Splitting of mica.
Quarter-wave plates are most easily made from mica, since it is easily
split to the thickness required.
The stock sheets
are split from clear mica plates.61 The starting sheet is trimmed
to about 3 inches square with sharp tin snips so as to have clean edges.
(The exact size of the starting sheet is immaterial.) One corner of the
starting sheet is then frayed out by rubbing it, and a clean dissecting
needle is introduced to divide the sheet approximately in half. A drop
of water is introduced in the cavity so produced.62 The mica
is then split all around the edges by working the needle along, point
first, at an angle of about 30°, so that the first cleavage starts
inside the boundary of the sheet. This avoids a terraced cleavage. After
the needle has gone around the circumference, a second drop of water is
introduced, and the plates are drawn apart. The water so facilitates cleavage
that the sheets may be separated almost as easily as the pages of a book.
This process is repeated until the thickness is approximately 0.036 mm
or as thin as desired. Each time, the sheet is divided so as to give two
sheets of approximately the same thickness.

Fig. 42. |
Mica gauges.63
A gauge may be made up as shown in Fig. 42. To make such a gauge the principal
directions are first marked on a starting plate. The thinnest possible
sheet is then split from the starting plate and cut up into strips about
1/4 inch wide. The strips are cut at an angle of 45° with the principal
directions. These strips are then cut to give rectangles with lengths
of 2 inches, 1-7/8 inches, 1-3/4 inches, 1-5/8 inches, and so forth. (See
Fig. 42.) The strips are next cemented (with balsam) between glass plates
as illustrated, care being exercised to see that none of the strips are
mounted upside down or rotated end for end. The steps so formed are then
indexed.

Fig.
43. |
The retardation per
step of the gauge is determined as follows: After the analyzer is set
for maximum transmission of the light, the gauge is placed on the mirror
of the Norremberg doubler (see Fig. 43) either parallel or perpendicular
to the azimuth of polarization. A sodium light should be used for illumination.
The index number of the step which gives opacity is noted. The step giving
opacity is a quarter-wave plate for the D lines. Other steps are
proportionately greater and less.
Using the gauge.
The gauge is used as follows: First, the analyzing Nicol of the Norremberg
doubler is set for extinction. The mica of unknown thickness is placed
on the bottom mirror of the doubler, with its principal direction making
an angle of 45° with the azimuth of polarization to give maximum transmission.
Then the gauge strip is laid on top of the mica so that it is either parallel
or perpendicular to the azimuth of polarization. At one of these orientations,
the steps show "interference" colors, and at the other, and proper one,
opacity is obtained for one or two of the steps. The calibration value
of the step which gives opacity corresponds to the retardation of the
mica sample. Interpolation may be required to make a delicate measurement.
Magnification
of lenses. The transverse magnification of a lens is the ratio of
image diameter to object diameter, or, expressed another way, it is the
ratio of transverse image displacement to transverse object displacement.
For a simple lens the magnification is given by the ratio of image distance
to object distance. For a system such as a spectrometer, which has a collimating
element (lens or mirror) with the object at or near its focal plane and
a telescope element also with the image at or near its focal plane, the
magnification produced is the ratio of the focal length of the telescope
element to that of the collimating element.
Another case, encountered
in a telescope, is that in which parallel light is received by the objective
and observed by an eyepiece adjusted so that its focal plane is very near
the focus of the objective. Here, the angular magnifying power is the
ratio of the focal length of the objective to that of the eyepiece.
The longitudinal
magnification of an image-forming system gives the ratio of the displacement
of the image along the optical axis to the displacement of the object.
In the case of a system composed of two lenses (or mirrors) with the object
and image at or near the respective focal planes of these elements, the
longitudinal magnification is given by the square of the ratio of the
focal lengths.
Other properties
of lenses. When a beam of parallel light is focused with a thin lens
on the optical axis, its focal length f is given by the expression
 (3)
(3)
where 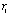 and
and  are the respective radii
of curvature of the two surfaces of the lens, and n is the index
of refraction of the material from which the lens is constructed. The
r's are taken positive if the curvature acts to converge the light. are the respective radii
of curvature of the two surfaces of the lens, and n is the index
of refraction of the material from which the lens is constructed. The
r's are taken positive if the curvature acts to converge the light.

Fig.
44. |
If the light is inclined
to the optical axis of the lens, it exhibits astigmatism as shown in Fig.
44. For example, the best focus of a distant star, which would be a small
hard spot of light on the optical axis, is a soft image when the lens
is inclined. The diameter of the smallest image is known as the "circle
of least confusion." Within the focal distance giving the smallest off-axis
image, the lens gives at one particular distance a rather sharp line focus,
which is perpendicular to the plane passing through the image and the
optical axis. Also, outside this image another rather sharp line focus
is obtained. This line focus is perpendicular to the first line and parallel
to the plane referred to above.
The astigmatism of
a simple lens is illustrated in Fig. 44. The locus of the inner astigmatic
images is a circle, a, having a diameter
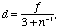 (4)
(4)
or 0.275 f
for n = 1.5, and the locus of the outer astigmatic images is a
circle, b, of diameter
 (5)
(5)
or 0.6 f for
n= 1.5.
Properties of
mirrors. The mirrors generally used in optics are conic sections of
revolution and the flat. They are paraboloidal for focusing parallel light,
ellipsoidal for two conjugate real focii, and hyperboloidal for two conjugate
focii, one of which is virtual. The spherical mirror is, of course, suited
for focusing light from a source at its center of curvature exactly back
on the center.
When a spherical
mirror of radius R is used to focus parallel light striking it
at an angle, the image exhibits astigmatism, and the lines corresponding
to the two circles shown in Fig. 44, determined by the positions of the
astigmatic images, are a circle of diameter R and a straight line,
respectively. (See Fig. 2l, Chapter XI.)

Fig.
45. |
Properties of
prisms. Some interesting properties of a right-angle prism are illustrated
in Fig. 45.
This prism, viewed
through the long face and perpendicular to the vertex of the 90•
dihedral angle in one azimuth, has the interesting and often useful property
of returning a beam of light back on its path, regardless of the angle
of incidence on the long face in the other azimuth. Fig. 46 illustrates
the corresponding property for the corner of a cube.

Fig. 46. |
Optical recording
systems. Professor Hardy has written an excellent article on recording
systems as applied to oscillographs.64 We can refer only to
his results. He concludes that a simple optical system with a single lens
in front of the galvanometer mirror will give as much illumination on
the recording film, on a basis of equal resolving power, as any other
possible stigmatic system. Furthermore, he points out that the focal length
of the simple systems should be chosen so that the limit to the resolving
power is set by the photographic material rather than by interference
effects. Although 25 lines/mm or more can be resolved by photography,
Hardy sets an arbitrary practical limit of 0.1 mm as the resolving power
of the photographic material. To obtain maximum illumination and at the
same time to conserve on the use of photographic materials, the simple
lens should be chosen to give a spot at least 0.1 mm wide.

Fig.
47. |
However, by using
an astigmatic optical system such as the one shown in Fig. 47, it is easily
possible to obtain nine times as much illumination as with the simple
lens. Furthermore, the astigmatic system has the additional advantage
that rotation of the galvanometer mirror about a horizontal axis does
not produce a vertical deflection of the image on the recording film.
The calculation of
the maximum velocity at which the recording spot can traverse the photographic
emulsion and still yield a perceptible trace is treated in Chapter XI.
This treatment includes the astigmatic case illustrated in Fig. 47. Owing
to the recent developments in fast photographic emulsions, the data given
in Table VI, Chapter XI, for the various materials may be regarded as
being distinctly conservative.
A bibliography of
some of the best works on the subjects treated in this chapter is given
in a footnote.65
1 The
autotransformer is as satisfactory as the battery when it is energized
by the output of a Raytheon voltage regulator.
2 These
lamps may be obtained from the General Electric Company, Nela Park, Cleveland,
Ohio.
3 This
lamp is supplied by the Philips Laboratory, Eindhoven, Holland.
4 Ives,
H. E., Kingsbury, E. F., and Karrer, E., "A Physical Study of the Welsbach
Mantle," Frank. Inst., J., 186, 401, 585 (1918).
5 Forsythe,
W. E., J. O. S. A., 7, 1115 (1923).
6 Rubens,
H., Deutsch. Phys. Gesell., Verh., 7, 346 (1905); Ann. d. Physik,
18, 725, (1905), 20, 593 (1906); Phys. Zeits., 6, 790
(1905), 7, 186 (1909).
7 Barnes,
R. B., Rev. Sci. Instruments, 5, 237 (1934).
8 Pfund,
A. H., J. O. S. A., 8ff, 439 (1936).
9Nernst,
W., and Bose, E., Phys. Zeits., 1, 289 (1900). Nernst glowers are
obtainable from Stupakoff Laboratories, 6627 Hamilton Avenue, Pittsburgh,
Pennsylvania.
10
Griffith, H. D., Phil.
Mag., VI, 50, 263 (1925).
11
For the theory of
the hydrogen ballast lamp, see Busch, H., Ann. d. Phsik, 64, 401 (1921).
12 I am
indebted to C. H. Cartwright for this information.
13 Benford,
F., Trans. Soc. Motion Picture Eng., 24, 71 (1926).
14 Kayser,
H., Tabelle der Hauptlinien der Linienspektra aller Elemente. Berlin:
Julius Springer, 1926.
15
See the following:
Babcock, Harold D., Astrophys. J., 66, 256 (1927), 67,
240 (1928). St. John, Chas. E., and Babcock, Harold D., Astrophys.
J., 46, 138 (1917), 53, 260 (1921).
16
Pfund, A. H., Astrophys.
J., 27, 298 (1908).
17 The
National Carbon Company produces a spectroscopic grade of pure I graphite.
The pure carbon are exhibits only one line in the visible or the ultraviolet
spectrum. This line is 2478 A.
18 For
a description of a simple, home-made, low-pressure arc, see Pfund, A.
H., Astrophys. J., :27, 299 (1908).
19 Baly,
E,. C. C., Spectroscopy. New York: Longmans, Green and Company,
1927.
20 This
lamp and the one discussed above are obtainable from the Cooper-Hewitt
Electric Company, Hoboken, New Jersey.
21 Harries,
W., and Hippel, A. v., Phys. Zeits., 33, 81 (1932).
22 This
lamp is obtainable from Schott und Gen., Jena, Germany. Their agent in
this country is Fish-Schurman Corporation, 250 East 43rd Street, New York.
23 Bol,
C., Das Licht, 5, 84 (1935); Ingenieur, 50, 91 (1935). Barnes,
B. T., and Forsythe, W. E., J. O. S. A., 27, 83 (1937). Dushman,
S., J. 0. S. A., 27, 1 (1937). A bibliography of high efficiency
light sources is given.
24 The
Bol lamp must be operated surrounded with a stream of cooling water.
25 This
lamp is obtainable from the Reed and Miller Company, 16 South Raymond
Street, Pasadena, California.
26 See
Leighton, W. G., and Leighton, P. A., Jour. of Chem. Ed., 12, 139
(1935).
27 For
wide monochromator slits, the tungsten lamp is a much richer light source
in this region than the argon discharge.
28 Buttolph,
L. J., Am. Illum. Eng. Soc., Trans., 30, 147 (1935). For similar
lamps using other metallic vapors, see Alterthum, H., and Reger, M., Das
Licht, 3, 69 (1933).
29 See
Chapter XIV.
30 Brice,
R. T., Rev. Sci. Instruments, 8, 209 (1937). Strong, J., and Brice,
R. T., J. 0. S. A., 25, 207 (1935).
31 Duffendack,
O. S., and Manley, J. H., J. O. S. A., 24, 222 (1934). Duffendack,
O. S., and Thomson, K. B., J. O. S. A., 23, 101 (1933). Herzberg,
G., Ann. d. Physik, 84, 553 (1926). Jacobi, G., Zeits. f. techn.
Physik, 17, 382 (1936). Lau, E., and Reichenheim, O., Zeits. f.
Physik, 73, 31 (1931). Lawrence, E. O., and Edlefsen, N. E., Rev.
Sci. Instrument, 1, 45 (1930). Munch, R. H., Am. Chem. Soc., J.,
57; 1863 (1935). Smith, A. E., and Fowler, R. D., J. O. S. A.,
26, 79 (1936).
32 These
tubes may be obtained from the Central Scientific Company, Chicago, Illinois,
and A. D. Mackay, 198 Broadway, New York City.
33 Wood,
R. W., Physical Optic, page 93. New York: The Macmillan Company, 1934.
34 For
a general treatment of ultraviolet radiations, see Luckiesch, M., Holladay
L. L., and Taylor, A. H., Frank. Ind., J., 196, 353 (1923).
35 Wood,
R. W., Phys. Rev., 44, 353 (1933).
36 O'Bryan,
H. M., Rev. Sci. Instrument, 6, 328 (1935).
37 Wood,
R. W., Phil. Mag., 6, 257 (1903).
38 Bäckström,
H. L. J., Naturwiss., 21, 251 (1933).
39 Land,
E. H., Frank. Inst., J., 224, 269 (1937). Freundlich, H., Chemistry
and Industry, 56, 698 (1937).
40 Kellner,
L., geb. Sperling, Zeits. f. Physik, 56, 215 (1929). The paraffin
in question is Kurlbaum, M. P., 68° to 72°C.
41 Bridgman,
P. W., Am. Acad., Proc., 60, 307 (1925), 64, 19 (1929).
Korth, K., Zeits. f. Physik, 84, 677 (1933). Kyropoulos, S.,
Zeit. f. anorg. allgem. Chem., 154, 308 (1926). Ramsperger, H., and
Melvin, E. H., J. O. S. A., 15, 359 (1927). Stober, F., Zeits.
f. Krist., 61, 299 (1925). Strong, J., Phys. Rev., 36, 1663
(19300.
42 Strong,
J., Phys. Rev., 37, 1661 (1931).
43 Nichols,
E. F., Ann. d. Physik, 60, 401 (1897); Phys. Rev., 4, 297
(1897). Rubens, H., and Nichols, E. F., Ann. d. Physik, 60, 418
(1897); Phys. Rev., 4, 314 (1897).
44 Absorption
of water: Nicholson, Seth B., and Pettit, Edison, Astrophys. J., 56,
295 (1922). Absorption of cupric chloride solution: Coblentz, W. W., Bureau
of Standards Scientific Paper No. 168.
45 Heat-absorbing
glass is manufactured by the Corning Glass Company, Corning, New York.
BG17 and BG19, manufactured by Schott und Gen., are handled in this country
by the Fish-Schurman Company, New York City.
46 Christiansen,
C., Ann. Physik u. Chemie, 23, 298 (1884), 24, 439 (1885)
McAlister, E.D., Smithsonian Misc. Coll., No. 7 (1935).
47
Weigert, F., Staude,
H., Elvegard, E., and Shidei, J., Zeite.f. phys. Chem., Abl. B, 2,
149 (1923), 9, 329 (1930).
48 Barnes,
R. B., and Bonner, L. G., Phys. Rev., 49, 732 (1936).
49 Pfund,
A. H., J. O. S. A., 14, 337 (1927). For a grating spectrometer
application of Pfund's scheme see Randall, H. M., Rev. Sci. Instruments,
3, 196 (1932). Hardy, J. D., Phys. Rev., 38, 2162 (1931).
50 Czerny,
M., and Turner, A. F., Zeits. f. Physik, 61, 792 (1930). Czerny,
M., and Plettig, V., Zeits. f. Physik, 63, 590 (1930).
51 Littrow,
O., Am. J. Sci., 35, 413 (1862). Wadsworth, F. L. O., Phil.
Mag., 38, 137 (1894); Astrophys. J., 2, 264 (1895).
52 Harrison,
George R., Rev. Sci. Instruments, 5, 149 (1934).
53 Rubens,
H., and Wood, R. W., Phil. Mag., 21, 249 (1911).
54 Forbes,
Geo. S., Heidt, Lawrence J., and Spooner, Lawrence W., Rev. Sci. Instruments,
5, 253 (1934).
55 Strong,
J., Phys. Rev., 37, 1565 (1931), 38, 1818 (1931).
56 Strong,
J., J. O. S. A., 26, 256 (1936).
57 Brahtz,
J. H. A., Rev. Sci. Instruments, 5, 80 (1934). Goetz, A., Rev.
Sci. Instruments, 5, 84 (1934).
58 Coker,
E. G., and Filon, L. N. G., A Treatise ort Photo-Elasticity. London:
Cambridge University Press, 1931; New York: The Macmillan Company, 1932.
Horger, O. J., Jour. of Applied Physics, 9, 457 (1938). This article
contains a good bibliography on the subject.
59 Einsporn,
E., Phys. Zeits., 37, 83 (1936).
60 Kaplan,
Joseph, J. O. S. A., 14, 186 (1927).
61 Mica
is obtainable from Eugene Munsell, 200 Varick Street, New York City.
62 Strong,
J., Rev. Sci. Inetrumente, 6, 243 (1935).
63 Wright,
Lewis, Light, page 289. New York: The Macmillan Company, 1892.
64 Hardy,
A. C., J. O. S. A., 14, 506 (1927).
65 Baly,
E. C. C., Spectroscopy. New York: Longmans, Green and Company, 1927.
Forsythe, W. E.,
Measurement. of Radiant Energy. New York: McGraw-Hill Book Company,
1937. Hardy,
A. C., and Perrin, F. H., The Principles of Optics. New York: McGraw-Hill
Book Company, 1932. Lecomte,
J., La Spectre Infrarouge. Les Presses Universitaires de France,
1928. Meyer,
Charles F., The Diffraction of Light, X-rays and Material Particles.
Chicago: University of Chicago Press, 1934. Schaefer,
C. L., and Matossi, F., Das Ultrarote Spektrum. Berlin: Julius
Springer, 1930. Wood,
R. W., Physical Optics, Third Edition. New York: The Macmillan
Company, 1934. |

![]()